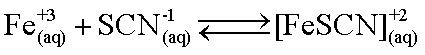
Experimentally Determined Equilbirum Constant
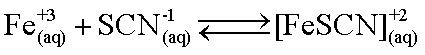
This reaction is an equilibrium, not
going completely to the right side.
A theater company (Bad Actors, Inc.) has
discovered this reaction and wants to use it to prepare fake blood
solutions. They need to know what the equilibrium constant is so they
can prepare the correct concentrations of KSCN and Fe(NO3)3
to get the needed red color.
Since the FeSCN+2 complex is colored we can use the Beer-Lambert law (A = εbc) to determine the concentration of FeSCN+2. If we know the initial concentrations of Fe+3 and SCN-1, and what the final concentration of FeSCN+2, we can determine the equilibrium concentrations of each species and calculate Kc for this reaction given.
A standard curve of known FeSCN+2 concentration vs. absorbance data must be determined. This will be done by making a set of standards with an extreme excess of SCN-1 ion (the stock solutions will be ~0.1 M SCN-1 and ~5.0 x 10-4 M in Fe+3, read the bottle for exact concentration.) which will drive the reaction essentially all the way to the right, making (for these 'known' runs) [FeSCN+2] = [Fe+3] These runs will NOT be used to determine K for the reaction, they are just a source of colored FeSCN+2 ion.
The stock solutions (both of them) for the formation constant determinations are ~2.0 x 10-3 M (get exact concentration from bottle)
You
must remember all you have learned regarding the driving of the
spectrometer. What the effective ranges are, how to make varying
concentrations of solutions, etc. Also, know that the iron solutions will
be acidic (~0.5 M HNO3) to prevent the
formation of other species that are colored.
You should probably re-read the experiment
you did last term on spectroscopy, it will refresh your
memory. What would be even better is looking at your lab report for
that experiment.
5
min screencast of experiment
Report: Your lab report consts of a filled out data sheet (see pre-lab question #0) along with your graph and initialed pre-lab. On the backside of the data sheet, give standard short form memo commentary section.
Prelab Questions
(Where should I write them??)
You will NOT turn this in but it must be complete
before you enter lab.
You will include it stapled to your report 1 week later. You must make
sure that the instructor has marked your prelab sheet, or a zero score
you will get.
0. Print out a copy of the Data Sheet for the experiment and bring it to lab.
1.Review the MSDS for potassium thiocyanate and briefly list the hazards.
2. NH4Cl(s)
<-->1 NH3(g)
+ 1 HCl(g)
~MEO 2 Apr 08