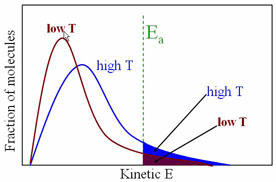
Effect of Temperature on rate of Reaction
The rate of reaction is a function of many things, including surface area, concentration and temperature. For a reaction to take place, the molecules must collide with enough energy (called the Activation Energy, EA) Consider the reactant molecules to be at a particular temperature. By definition, the molecules have an average kinetic energy, some with more than the average, some with less. If we increase the temperature of a system, the average kinetic energy will increase and the number of molecules with the 'minimum' energy to react, thus increasing the frequency (rate) of reaction.

Svante Arrhenius has shown that for simple reactions, the equation
k
= Ae-Ea/RT →
ln k = -EA/RT
+
ln A
where k = rate constant, A is the collision frequency factor (function of the nature of reaction) R is the gas constant, T is the absolute temperature, EA is the activation energy, and e is the base of the natural logarithms. We want to know the relationship between rate of reactions and two different temperatures. After some creative algebra, we can transform the Arrehenius equation into something manageable:
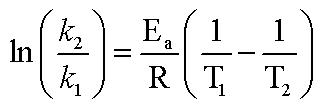
1
H2O2
+ 2 HI → 2 H2O + 1 I2
This reaction has been shown to be a second order reaction, first with respect to [HI], first with respect to [H2O2]. The experimentally determined rate law is of the form:
rate
= k[HI][H2O2]
The total volume of the solution containing the components of the reaction is kept constant during the course of this experiment. In fact, we keep everything constant for each run EXCEPT the temperature, so for our purposes, we can write the rate law as: rate = k (constant)
Since, in our equation we are looking at the ratios of the rate constants, and rate is directly proportional to rate constant (in our system here only because we are keeping everything constant) That means, instead of using the ratios of k's, we can use the ratios of rates instead.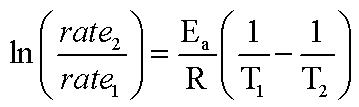
Starch is added to this solution as the indicator of free iodine. We start the stopwatch at the moment we add our second reactant, H2O2. Of course the production of I2 begins immediately, and the blue color will instantly appear. A measured volume of 0.1 M Na2S2O3 is then added, causing the blue color to disappear due to the reduction of the I2 by thiosulfate. The solution will stay colorless until enough additional I2 is produced by H2O2-HI oxidation to react with the remaining Na2S2O3 in the solution. At this point the blue color will reappear. The time is noted (stop stopwatch) This represents the time required to produce I2 equivalent to the Na2S2O3 in the volume of 0.1 M Na2S2O3 which was added. The entire reaction will be repeated with the same concentrations, but at a different temperature.
Wow, that is confusing. You probably wanna watch the explanation screencast a few times.
Chemicals needed- 0.60 M KI (freshly made) , 0.100 M Na2S2O3, 0.6% H2O2 (freshly made from 3% soln), starch solution, 6M H2SO4, ice
2. Add 10.00 mL
of KI using a volumetric pipet to the rxn beaker alone with ~200 mL of DI water.
3. Add 15 mL of 6M
H2SO4 (caution!) and
carefully place the beaker in the
1L water (or ice for the low temp run) bath beaker. Place a stir bar in the solution and place
the
entire system on a stir plate and turn it on (no heat!!)
5. Add 5.00 mL of 0.6% H2O2 solution (using a volumetric pipet) and place
it in a test tube, stopper it, and put it in the rxn beaker.
6. You want to wait until everything is at the desired
temperature before you go any further. Once you start the reaction, you
will
want to keep the system stirring to keep everything at the same (water
bath)
temperature.
7. Add some starch indicator to the rxn beaker. Your instructor will tell you how much.
8. Add the H2O2 to the
solution and
begin timing. The solution should immediately turn blue.
That’s a good thing.
The stopwatch does not get stopped until that blue color returns (you are about to make it go away)
9. WITHIN 30 SECONDS, Add 4.00 mL of 0.100 M Na2S2O3 to the rxn beaker using a volumetric pipet.
10. Stop the stopwatch as soon as the solution STARTS to turn the blue/brown color again.
Three good runs at room T, calc rate, calc k for each run, average the three k's to get ONE k for that T.
One good run at near ice T (rxn temp needs to be lower than 4 C), calc rate, calc k
Calc Ea from the two T's, and the two k's.
Prelab:
1. The decomposition of
ethylene oxide at 652 K is the first-order reaction with k = 0.0120 min-1 and
an activation energy of 218 kJ/mol. What is the RATE OF REACTION at 752 K?
(CH2)2O(g) → CH4(g) + CO(g)
You will also need to show the instructor your premade computer generated data
sheet
before you step foot in the lab. No computer generated data sheet no
lab for
you!