Gasoline Additive
Unleaded gasoline currently contains compounds known as oxygenates which are responsible for increasing the octane rating and reducing engine 'knocking'. Oxygenates also reduce carbon monoxide emissions. MTBE and ETBE (Methyl-t-Butyl Ether and Ethyl-t-Butyl Ether) are examples of oxygenates along with methanol and ethanol. Ethanol will increase the the octane rating approximately 1 unit for each 3% ethanol added. Research with pure alcohol fuels show better performance with ethanol. In addition, the current political climate with respect to farm-grown fuels favors ethanol as gasoline additives.
Consider a Midwestern producer of ethanol for fuel has just been approached by an entrepreneur who is considering a specialty gasoline additive product. The approximate composition of this product is 80% ethanol and 12% t-butanol (also an additive to gasoline and used as a gasoline de-icer) and 8% other additives. The ethanol is available locally (Lucky's Bar) and is handled as a liquid. The t-butanol however will be shipped from a petrochemical producer. The t-butanol is usually handled as a liquid, shipped in bottle, solvent drums and tank cars. However, it has a freezing point of ~25 deg C. All containers of this material will need to be heated and melted during the fall, winter and spring months which would be way expensive. An engineer has suggested adding some ethanol to the t-butanol (you are going to add some later anyways to make your additive) by the manufacturer to reduce the freezing point to keep it from freezing. You have been asked to determine how much ethanol should be added to keep the t-butanol-ethanol mixture a liquid down to -10 F. You have an equation for calculating new freezing point of a solution. All you need to answer the questions is the freezing point depression constant.
The easiest way to do this is to
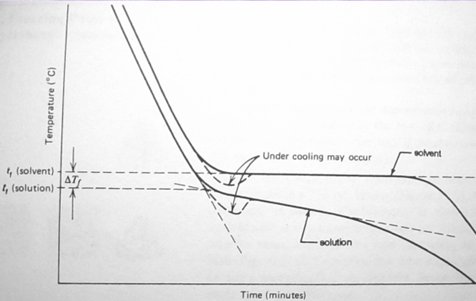
determine the freezing point of pure t-butanol, (see cooling curve below) then
add a little ethanol. Measure the new freezing point. The freezing points of the solvent
and the solution are obtained from a cooling curve, a plot of temperature vs.
time. An ideal plot looks thusly:

The cooling curve for the pure
solvent reaches a plateau at its freezing point. Extrapolation of the curve for
the solution to the temperature axis determines the freezing point. The cooling
curve for the solution does not reach a plateau, but continues to decrease as
the solvent slowly freezes out of solution (why?) Its freezing point is
determined at the intersection of two straight lines drawn from above and below
the first bend in the cooling curve (see figure). From this data we can
determine the freezing point of the solution.
Add some more ethanol, measure the freezing point again. Repeat. You now have some data for change in freezing point and associated molalities. The equation for freezing point depression, ΔTf = Kfm shows that the change in freezing point varies linearly with the molal concentration. A graph of ΔTf (y axis) vs. molal concentration should therefore give a straight line with a slope equal to the freezing point depression constant. Calculations can then be done for the report (how much ethanol added per kg of t-butanol to get a freeing point below -10 F).
Disposal: All solutions go in Waste 't-butanol solutions' container, NOT down the drain.
Report: This is a formal lab report, complete with introduction, experimental, results and discussion sections. You will be submitting computer-generated graphs only (with equations) with the final report. Your graphs can be small enough that you fit them all on the same piece of paper. You need not include pictures of your cooling curves, but the ΔTf vs. molal concentration graph is needed (with equation and R2 value.)
Before you enter the lab, you must show the instructor a brief summary of the hazards of t-butanol (also known as tert-butanol, or 2-methyl-2-propanol) from the MSDS. Additionally, you must present a piece of recycled paper with places for ALL the data you will be taking that day. If you do not have these things, you will not be doing the lab that day.
~JWS/MEO 1 Feb 06