Last Experiment : The Chemistry of Tie Dye
DOUBLE CHECK WITH YOUR INSTRUCTOR AS TO WHAT TIME LAB STARTS!!
Background:
The art of dying probably originated in India or China no later than 2500 BC Most natural dyes came from parts of plants such as the bark, berries, flowers, leaves, and roots. Because these dyes did not have a strong attraction to the fibers being dyed, a process known as mordanting was used to improve colorfastness. To react with acidic dyes, fibers were treated with basic or metallic mordant that might contain solutions such of aluminum, copper, iron, or chromium salts. Compounds formed by the dye and mordant, called lakes, prevented the colors from washing out and made the color last longer. Such natural dyes became less and less important as synthetic dyes that produced brighter colors were developed. Today, logwood black is the only natural dye widely used.
In 1856, William Henry Perkin began the synthetic organic chemical industry by accidentally discovering the purple dye mauveine when he was trying to make quinine from aniline. The synthetic dyes were known as coal tar dyes because of the six membered ring structures of carbon atoms were all derived from coal tar. Congo Red was the first dye discovered with so great an affinity for cellulose that a mordant was not required.
About 100 years after Perkin's first discovery, fiber-reactive dyes capable of forming covalent linkages with the fiber were discovered. A fiber-reactive dye is washfast. During dying, dye molecules must diffuse from aqueous solution into the fibers. Fibers such as cotton absorb water readily and are said to be hydrophilic, while fibers such as polyester absorb water with difficulty are described as hydrophobic. Dyeability is influenced if a fiber can somehow carry an ionic charge and better interact with oppositely charged colored ions. To dye cellulose, a reactive dye must combine with hydroxyl groups in the fiber.
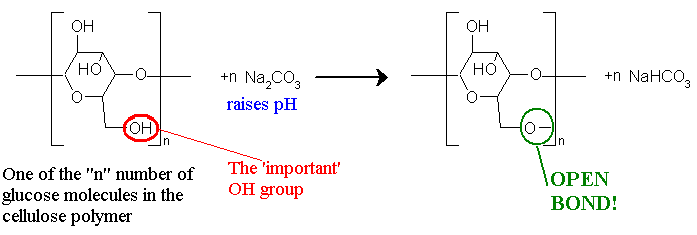
In this lab you wi1l be actually doing a chemical experiment as the fibers of the your garment react with the molecules of dye. The chemistry involved is actually relatively straightforward. The cotton fabric which is primarily cellulose wi1l be soaked in a solution of Na2CO3 which is a weak base, which raises the pH and creates a oxygen atom with only one single bond to the rest of the molecule, which is quite reactive:

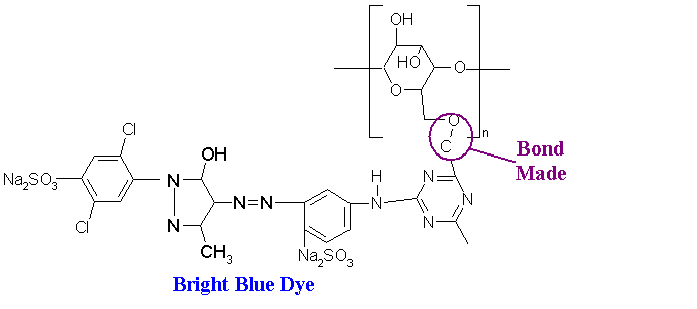
It is this open bond that wi1l chemically bond with the particular dye molecule. The example below shows how the cellulose interacts with a molecule of blue dye.
Tie dying is one of the oldest methods of
printing designs on fabrics. Typically, parts of the fabric are bunched
together in a design an knotted or tied together with string. Tied
sections are protected from absorbing the dye and patterns are created.
Procedure:
A. Tieing
1. Label your shirt with your initials and place it in the NaCO3
solution for >10 minutes.
2. Put on a pair of gloves and an apron if you do not want to stain
your clothes. Get the shirt from
the sodium carbonate solution and wring it out.
3. After you choose the fold you want, commence folding, holding the
folds in place with rubber
bands. Be sure to fold carefully, the better the folding, the better
the finished product.
B. Dyeing
1. Take your tied shirt and place it on some clean newspaper. Using the
supplied bottles, start
applying dye to the section of the shirt carefully until you notice the
dye is being absorbed very slowly.
2. As you apply other dyes, do not touched dyed areas of the shirt with
your hands. This will spread the dye to undyed portions of the shirt
and contaminate those areas. Be sure not to lay the shirt in puddles of
dyes on the newspaper. This will also contaminate the dye.
3. Do not put complimentary colors near each other. as they will
combine to form icky colors.
4. When finished dying your shirt, wrap the dyed shirt in lots of fresh
newspaper and place inside
a 1 gallon plastic ziploc bag and seal it.
C. Washing
1. The first wash is the most important. After >24 hours, open
the bag and unwrap the shirt in a bathtub. Remove rubber bands and
rinse the shirt in hot water until the shirt no longer feels soapy (pH
neutral). You have no deactivated the glucose and excess dye. Rinse all
dye down the drain. This dye will not stain the tub, but will stain
carpet or rugs.
2. Get the shirt to a washing machine and wash with dish soap (two
tablespoons) by itself. DO not,
use laundry soap or dishwasher soap as this will reverse the reaction.
3. Dry the shirt on cotton setting in the dryer. In subsequent washing,
wash with colored clothes
only. Wash with regular laundry detergent and color safe bleach only.
Prelab questions/procedure: (on a separate piece of recycled
paper)
1. What shape are you planning on attempting to form? Briefly describe
how you will make it. You need to do some research!
2. Find the chemical formula of two different synthetic dye colors.
3. Why are you wearing grungy clothes today in lab?
4. Review the MSDS for sodium
carbonate. Give a brief overview of the hazards.
5. To get the tie to 'take' really well, hand wash the garment in dish soap (then dry) before you come to lab,
6. Bring a big pile of old newspapers (if you can) to cover the lab countertops.
~MEO 24 Apr 06