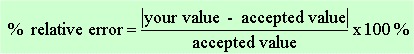
Purpose
To derive an experimental value of R and compare it to the published value. A known mass of a metal is reacted with hydrochloric acid to form hydrogen gas, H2. The volume, pressure, temperature and number of moles of the gas are calculated, allowing for the calculation of R.
Introduction
The ideal gas law, PV=nRT can be used to calculate the relative measure quantities of a sample of gas. Knowing three of the four measured quantities (n,P,V and T) we can calculate the fourth if R is known. In this lab we will experimentally determine R by generating a sample of gas and measuring the four quantities and calculating the gas constant by two methods. The procedure for this experiment is based on the chemical reaction between Mg and HCl to produce H2(g):
1 Mg(s) + 2 HCl(aq) ---> 1 MgCl2(aq) +1 H2(g)
The exact mass of Mg will be measured and using the above equation, the moles of H2 produced can be determined. (The reaction goes to completion) The experiment is done in a gas collection tube which is calibrated, so the volume will be easily read. Before measurements are taken, the gas system will be left to equilibrate to the ambient room temperature which can thus be easily measured. Determining the pressure however is a little more challenging. The hydrogen is collected in a eudiometer tube over an aqueous solution and thus after the reaction is complete, the pressure of the gas is that of the produced hydrogen and the vapor pressure of water (which is easy to find in a table somewhere)
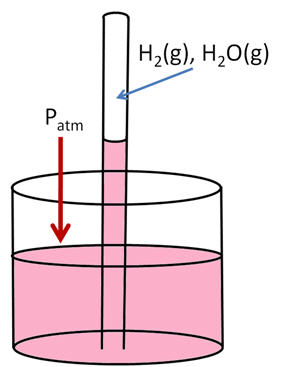 The
liquid levels are the same at the suface of the beaker water, so the
pressures on each side are the same. Atmosperic P on one side, H2(g), H2O(g) and a column of water on the other side: Patm = PH2(g) + PH2O(g) + PH2O(l)
The
liquid levels are the same at the suface of the beaker water, so the
pressures on each side are the same. Atmosperic P on one side, H2(g), H2O(g) and a column of water on the other side: Patm = PH2(g) + PH2O(g) + PH2O(l)You know the pressure of the atmosphere, you look up vapor pressure of water, and you measure the difference in P between the inside of the tube and the atmosphere, as measured by a ruler (PH2O(l) ). Unfortunately, you measured P in mm of H2O, not mm Mg. To convert the pressure difference in terms of mm of Hg, you must divide mm H2O by 13.546 since Hg is 13.546 times as dense as water at room temperature.
Solve of PH2(g) and this is called the ‘dry’ pressure of H2(g) since there is no H2O(g) included in the pressure value. Make sure when you use this equation that all the units are the same (mm Hg).
PV=nRT is
called the ideal gas law as it assumes some fundamental properties of gases,
namely that the gas molecules have no volume and that their attractive forces
are zero which is not always a valid assumption. You will be
calculating the value of R using PV=nRT. You will also need to
calculate the relative error obtained in your experiment:
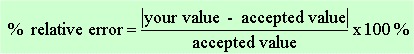
Prelab questions: (To be written on separately on a piece of recycled paper)
1. What is the vapor pressure of water at 25 deg C ? Where did you get this information?
2. Calculate the value of R using the ideal gas law for the following data: Vdry H2 = 73.52 mL ; Pdry H2 = 635 torr ; massMg (which gets you moles of H2) = 0.0811 g ; T = 23.5 °C.
3. Your instructor will show you how to do setup the experiment, but you must know what information you need to determine for your three runs. Come up with some semblance of a data sheet. You will show this to your instructor on the way in. No data sheet, no lab.
~MEO 23Oct 2006