Cheap Chemical Synthesis
A local chemical producer (OttChem, Inc.) specializing in hard-to-make solids has lost one of their key lab rats and no longer has the ability to produce calcium carbonate. Instead of trying to figure out how the person made it, they decide to subcontract out their calcium carbonate synthesis business to you and your partner. Calcium carbonate is a valuable pharmaceutical used to help prevent osteoporosis, treat upset stomach, and has demonstrated limited value in treatment of chemlabgraditus.
As an independent chemical company you must buy the raw materials for your synthesis. The local supply house will be played by your instructor. A vial will be given to you to extract from and you must return the after you have weighed it.
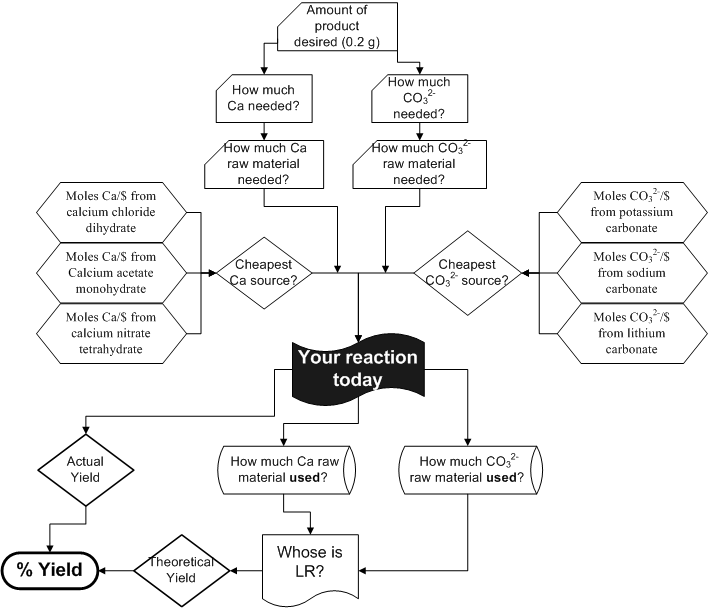
OttChem, Inc. is prepared to pay top dollar ($2723/g) for up to 0.2 g of calcium carbonate. Any more than that, you get to 'keep', and not get paid for. Your net profit is simply the amount you get paid for the product minus the total cost of materials.
You will be given a current price list of stock chemicals when you get into the lab. The chemicals available (with current per-g prices behind) will be Calcium chloride dihydrate($600.), (see the bottom of pg 534 of your text for an explanation of hydrates) calcium nitrate tetrahydrate($333.), calcium acetate monohydrate($480.), potassium carbonate($155.), lithium carbonate($225.), and sodium carbonate($195.). There are 9 possible combinations of these 6 chemicals that will give calcium carbonate as a product. Which is the most cost effective is a question you must answer.
All
of the Ca raw material will react the same, as will all of the
carbonate raw materials, so we can write a generic reaction for the day:
1 CaX·YH2O (aq) + 1 Z2CO3( aq) --> 1CaCO3(s) + other stuff

For this lab there is no formal lab write up or report, but you must fill out the data sheet. You must print out the data sheet before you come to lab, if you don't well, then I guess you will be writing up everything by hand. That would suck.
Pre-lab questions (on a separate piece of recycled paper and checked at the beginning of lab )
1. Give the molecular formulae and molecular weight of each of the 6 chemicals available.
2. Determine what you believe to be the cheapest pair of chemicals to use for making calcium carbonate. A hint would be to figure out how much of the ion desired (Calcium or carbonate respectively) you get for $1 of each raw material. (for example $1 calcium nitrate tetrahydrate -> g calcium nitrate tetrahydrate -> moles calcium nitrate tetrahydrate -> moles Ca)
3. Review the MSDS for calcium carbonate and give a brief overview of the hazards.
~MEO (with many thanks to JWS) 10.28.09