Four
Piece Seperation
Most of the matter we encounter in everyday life consists of mixtures
of different substances. Mixtures are combinations of two or
more substances in which each substance retains its own properties.
Mixtures are characterized by two fundamental properties that each of
the substances in the mixture retains its chemical identity and
mixtures are separable into these components by physical means.
If one of the substances in a mixture is preponderant that is, if its
amount far exceeds the amounts of the other substances in the mixture
then we usually call this mixture an impure substance and speak of the
other substances in the mixture as impurities.
Although there are numerous physical properties that can be used to
identify a particular substance, we will be concerned in this
experiment merely with the separation of the components and not with
their identification. The methods we will use for the separation depend
on differences in physical properties, but we will also exploit the
different chemical properties.
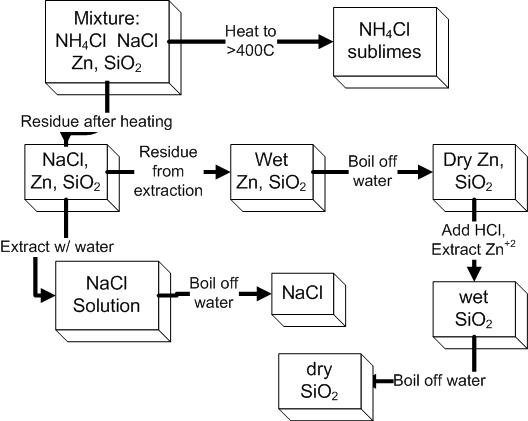
The mixture that you will separate contains four components: NaCl, NH4Cl,
Zn, and SiO2. Their separation will be
accomplished
by heating ~0.1g of the mixture in an evaporating dish to sublime the NH4Cl. The remaining solid (containing NaCl,
Zn, and SiO2)
extracting the NaCl with water, and then drying the remaining Zn/SiO2
mixture.
The Zn & SiO2 will be separated by adding an amount of HCl that will
react with the Zn(s) forming Zn2+(aq). This aqueous ion can easily be
'washed away' (extracted with distilled water, not even recovered) leaving the chemical inert sand.
Weighing the remaining sand will give yield the mass of Zn and SiO2 in
the original sample.
Lab picture:
Pre-Lab Questions
(recycled paper, to be seen and graded on the way into lab)
1.
Suggest a way to determine whether a colorless liquid is pure water or
a salt solution without tasting it.
2. Define the process of
sublimation, decantation, and filtration.
3. How do
decantation and filtration differ? Which should be faster?
4.
A student found that her mixture was 13.1% NH4Cl,
17.9% NaCl, 41.4% Znand 34.6% SiO2. Assuming her
calculations are correct, give two possible causes for her 'error'.~MEO 3.25.10
