Three
Piece Seperation
Most of the matter we encounter in everyday life consists of mixtures
of different substances. Mixtures are combinations of two or
more substances in which each substance retains its own properties.
Mixtures are characterized by two fundamental properties that each of
the substances in the mixture retains its chemical identity and
mixtures are separable into these components by physical means.
If one of the substances in a mixture is preponderant that is, if its
amount far exceeds the amounts of the other substances in the mixture
then we usually call this mixture an impure substance and speak of the
other substances in the mixture as impurities.
Although there are numerous physical properties that can be used to
identify a particular substance, we will be concerned in this
experiment merely with the separation of the components and not with
their identification. The methods we will use for the separation depend
on differences in physical properties.
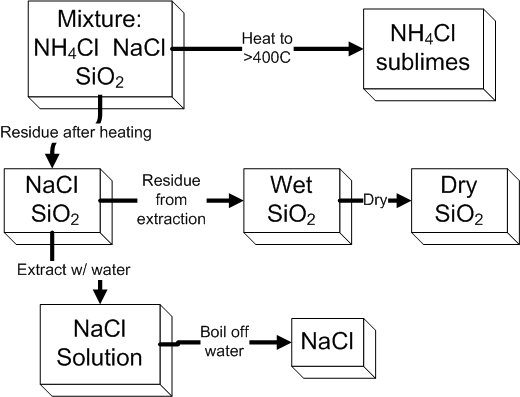
The mixture that you will separate contains three components: NaCl, NH4Cl,
and SiO2. Their separation will be accomplished
by heating the mixture to sublime the NH4Cl,
extracting the NaCl with water, and finally drying the remaining SiO2.
Lab picture:
Pre-Lab Questions
(recycled paper, to be seen and graded on the way into lab)
1.
Suggest a way to determine whether a colorless liquid is pure water or
a salt solution without tasting it.
2. Define the process of
sublimation, decantation, and filtration.
3. How do
decantation and filtration differ? Which should be faster?
4.
A student found that her mixture was 13% NH4Cl,
18% NaCl, and 75% SiO2. Assuming her
calculations are correct, what did she most likely do incorrectly in
her experiment?~MEO 11.13.09
